Research focus
ColoPredict Plus 2.0 Register Study
The molecular registry Colopredict Plus 2.0 – a platform for target identification and precision oncology in early colon carcinoma.
Through the close cooperation between the affiliated oncology clinics and the Institute of Pathology, structures have been created in recent years that make a significant contribution to the translation of research into clinical projects.
The central project of clinical and translational research is the non-interventional, multicentre Colopredict Plus 2.0 registry study. The registry is managed by the Institute of Pathology, Prof. A. Tannapfel, and coordinated jointly with Oncology (Prof. A. Reinacher-Schick). The project was started in 2013 and has so far been able to include over 5000 patients. In the meantime, the registry, which was initially started regionally, has been expanded nationally and currently includes 175 actively recruiting colorectal cancer centres throughout Germany. In addition to the collection, analysis and storage of tumour tissue samples, the registry records baseline characteristics and the clinical course over 5 years.
Due to the successful recruitment and the new possibilities of precision oncology, the study was expanded in 2018 to a perspective of 200 centres and 8000 patients. A new addition is the establishment of a biobank for blood samples, which will be used to identify and validate new prognostic and predictive markers from both tissue and blood.
The registry study also serves to record the current treatment and care reality in Germany, with a focus on the German Cancer Society’s colorectal centres, and thus contributes in perspective to improving treatment for colorectal cancer patients in the entire region and nationally.
Resulting publications:
Reinacher-Schick et al, Ann Oncol 2018 Suppl.
Nöpel-Dünnebacke et. al, Z Gastroenterol. 2020 Jun;58(6):533-541
Nöpel-Dünnebacke et al, Ann Oncol Suppl. 4, S415, Sep 01 2020
With an exploratory objective, it is also being investigated whether molecular prognostic signatures can be identified for patients in the above-mentioned stages, which enable a better prediction of entity-specific mortality and treatment success. This is done in cooperation with biophysics and proteome sciences as well as in close collaboration with experimental pathology in ProDi and bioinformatics in ProDi. Next-Generation Sequencing (NGS) with a panel specifically adapted to colon carcinomas is used to analyse the patient tissue samples (currently Quiagen HCCP panel with 96 genes). This enables patients to be identified on the basis of specific genetic or other molecular tumour characteristics for possible further therapy studies. In parallel, the complementary development of a Liquid Biopsy Biobank is being driven forward.
This enables us to actively participate in the planning of further cooperative national (via the Association for Internal Oncology (AIO) in the German Cancer Society) and international projects such as the cooperation with ALLIANCE and the planned cooperation with the British FOxTROT group.
Further basic research projects are taking place in particular in cooperation with the Institute of Pathology in ProDi.
The Colopredict Registry thus now represents an ideal screening platform for ongoing and planned interventional therapy studies according to the AMG and thus offers an optimal infrastructure in the age of precision oncology and the treatment of the “small groups”, i.e. the subgroups of patients defined by molecular markers, which in some cases make up only a few percent of the total collective. The study activity is carried out in cooperation with the AIO in the German Cancer Society as well as in cooperation with the Working Group of German Bowel Centres ADDZ. This innovative central screening option is unique to date and, in times of ever smaller subgroups and targeted therapy approaches within a tumour entity, will presumably not only enable better scheduling of clinical projects, but also targeted allocation of patients to the corresponding studies.
It is our conviction that the structured and standardised procedures will lead to considerable progress in quality (including analytical methods, central eligibility screening, verification of diagnosis) and quantity of recruitment. A reduction of avoidable costs is also likely (e.g. redundant analyses, data collection, etc.) and will thus also improve the feasibility of studies.
Last but not least, regulatory hurdles, such as contractual aspects, should also be reduced for future studies through the already existing networks and enable a faster and less complicated implementation of projects. Due to the current medical development of increasingly differentiated therapy options, the structure of the Colopredict platform will be forward-looking and strengthen Germany as a study location.
This will also give smaller and peripherally located hospitals cooperating with the iTZ Ruhr and the RUCCC the opportunity to provide patients with molecular diagnostics and to offer their patients interventional therapy concepts. Neoadjuvant therapy concepts are also discussed. The organisational and research structures listed above have ultimately led to the ATOMIC study, as the first study in Germany funded through the National Cancer Institute (NCI), to screen and recruit patients via Colopredict Plus 2.0. Prof. Dr. Reinacher-Schick was appointed as head of the clinical trial (LKP) here.
Other interventional study projects that integrate Colopredict Plus 2.0 into the screening process are.
- CIRCULATE (LKP Prof. Folprecht, Dresden). Evaluation of adjuvant therapy in stage II colon cancer after ctDNA determination (CIRCULATE), AIO-KRK-0217; EudraCT No. 2018-003691-12.
- NeoBRAF study (in submission, LKP PD Dr. Stein, Hamburg
- AIO-KRK-0220 ANTONIO (adjuvant treatment for high-grade microsatellite instability).
- ATOMIC study (AIO-KRK-0317/ A021502, EudraCT-No.: 2019-003562-40 (see above, LKP Prof. Reinacher-Schick)
With increasing understanding of the molecular pathological processes and the heterogeneity within a tumour entity, targeted therapeutic interventions and individualised risk stratification in patients are foreseeable in the near future and can, especially in the field of gastrointestinal neoplasms, be significantly influenced by the structures listed above.
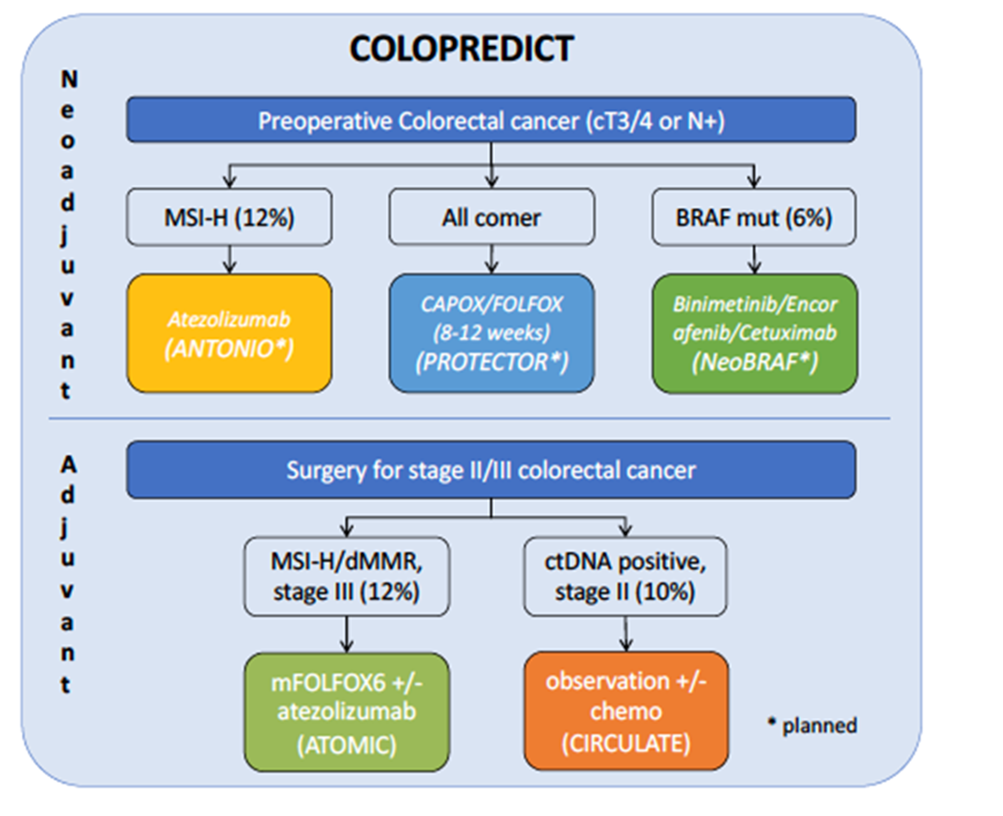
Fig. 1: Planning national and international projects with screening via the Colopredict (CPP) platform

Linking of new methods in clinical application (translational research).
In the competence area of biospectroscopy, characteristic vibration spectroscopic biomarkers are identified in order to automate the diagnosis and classification of oncological disease patterns. Due to its high sensitivity and the cumulative detection of all substances contained, a vibration spectrum represents a complete biochemical image – like a fingerprint – of the tissue sample.
Spectroscopy in cancer research
Tissue samples are analysed using IR (InfraRed) and Raman spectroscopic methods. Both spatially resolved microspectroscopic approaches and point measurements with fibre probes are performed.
This automated and sample-friendly methodology also allows diagnostics on small biopsies without destroying the sample for subsequent investigations, such as proteome analyses (Großerüschkamp et al Scientific Reports 2017). The focus of research in the biospectroscopy area of competence is on intestinal, bladder and thoracic tumours (e.g. lung cancer).
Translational research encompasses research activities that promote the integration of basic research, patient-oriented research and population-based research. In order to implement these goals, the method of biospectroscopy is planned in the TRANSLATIONAL RESEARCH WORKING GROUP as one of the central methods in the translational support programme for clinical studies. The aim is to validate the method across entities and to establish it in practical application in the future.